Draw a diastereomer for each of the following compounds. L-threose the enantiomer of D-threose has the R configuration at C 2 and the S configuration at.

Solved Draw A Diastereomer For Each Of The Following Chegg Com
Determine the relationship in each of the following pairs.
. Draw the enantiomer of the following compound. Start your trial now. The miso compound is one in which it has a mirrored plane is one way to look at it within the compound but also its an ant.
- Part A HO CH Foych This. 2 Enantiomer Diastereomer Feedback When drawing an enantiomer. Diastereomers are stereoisomers that are not superimposable and are not mirror images of each other.
First week only 499. By definition two molecules that are diastereomers are not mirror images of each other. Do the drawings represent constitutional isomers or.
A Pt NH32 SCN2 b CoCl2Br2 2 asked Oct 11 2019 in Co-ordinations compound by KumarManish. Humor is the same conference so those are the two ways to. Draw a diastereomer for each of the following compounds.
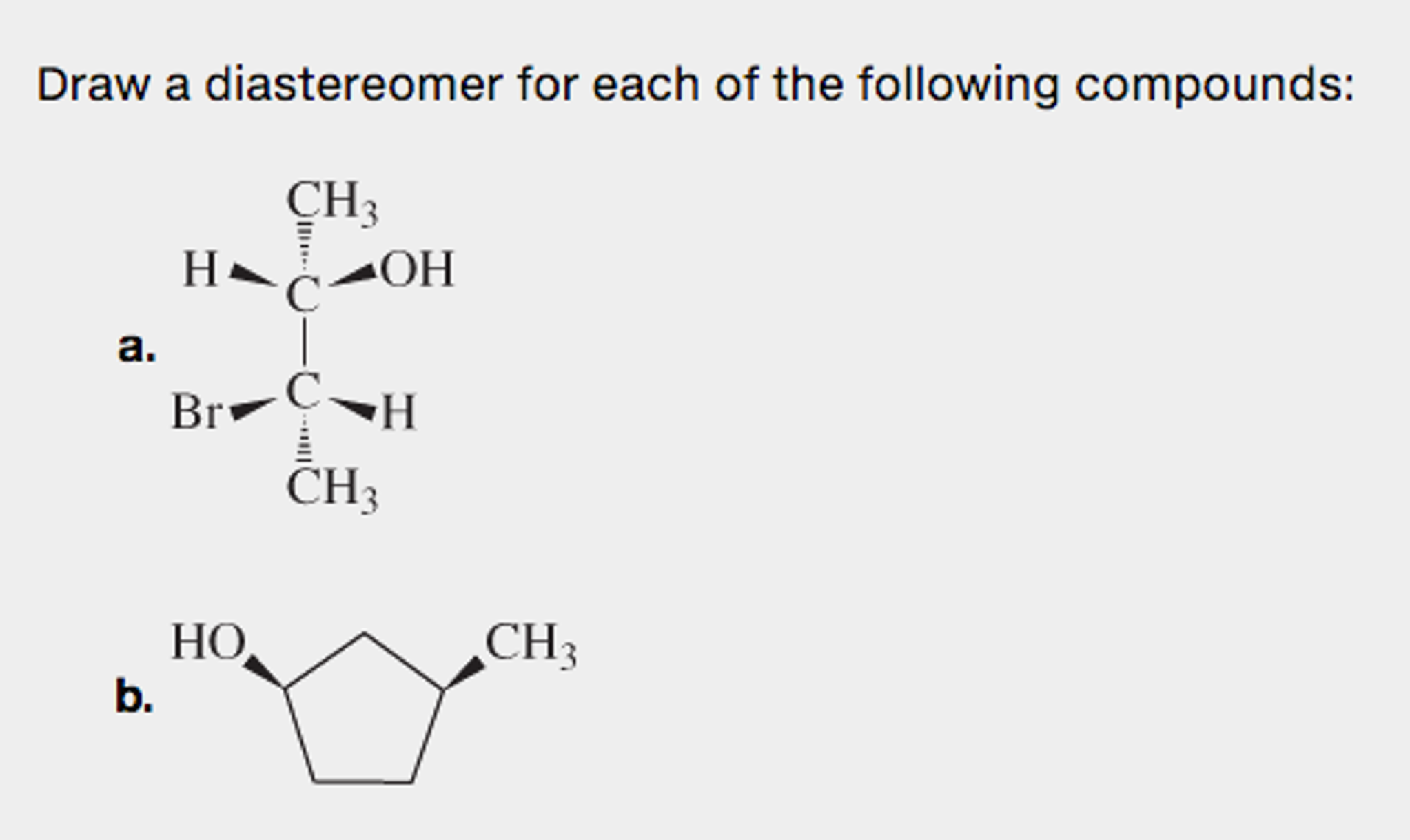
CH3 H OH CH3 CI CI Hw CH CH3CH2 CH3 H3C CH3 с с CH. Draw the enantiomer and a diastereomer for the following compound. Show the appropriate stereochemistry b- Н.
Part A CH3 H 2OH Br H CH3. Because the connectivity of atoms is the same and the arrangement is different these are stereoisomers. CH3 H OH CH3 CI CI Hw CH CH3CH2 CH3 H3C CH3 с с CH.
Part A CH3 H OH -ОН CH3 H Part B СІ ÇI H C-C-H CH3CH2 CH3 CH Part C HC CH3 CC H H Part D Draw the molecule on the canvas by choosing buttons froi active by default. Draw compound is a entantiomer and diastereomer following compound. How many geometrical isomers are possible for each of the following complexes.
Draw the enantiomer of the following compound. - which is mirror image of one another question_answer Q. Question Draw any diastereomer.
Draw a diastereomer for each of the following compounds. The best example for this is the cis and trans or E and Z isomerism. Draw a diastereomer for each of the following compounds.
For instance lets look at the following two molecules. A compound must have at least two chiral centres to have. Draw a diastereomer of the following compound.
For each of the following structures1Draw a Lewis structure. Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template. All are electrically neutral.
Fill in any nonbonding electrons2. To do this we will keep the configuration at one Ace Metrix under the same. Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template.
We have to draw diced area MERS with bowling compounds. Solution for Draw a diastereomer for each of the following compounds. Question Draw a diastereomer for each of the following compounds.
Draw the enantiomer and a diastereomer of the compound below. Draw a diastereomer for each of the following compounds. By signing up youll get thousands of step-by-step solutions to your homework.
Draw a diastereomer for each of the following compounds. And then at the. Calculate the formal charge on each atom other than hydrogen.
Draw a diastereomer for each of the following compounds. - Part A HO CH Foych Question. The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other.
Non superimposable mirror images are called enantiomers If R. Draw a diastereomer for each of the following compounds. HO HO OH OH OH OH edit structure.

Solved Draw A Diastereomer For Each Of The Following Chegg Com
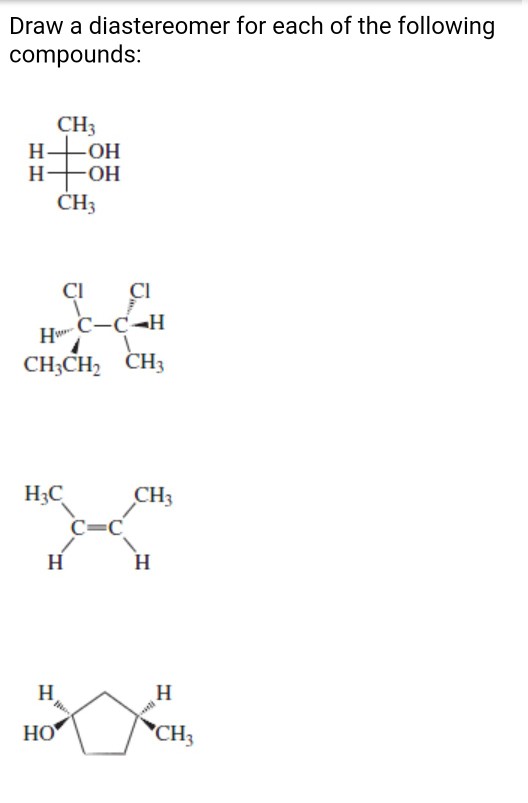
Solved Draw A Diastereomer For Each Of The Following Chegg Com

Draw A Diastereomer For Each Of The Following Compounds Study Com

Draw A Diastereomer For Each Of The Following Compounds Study Com
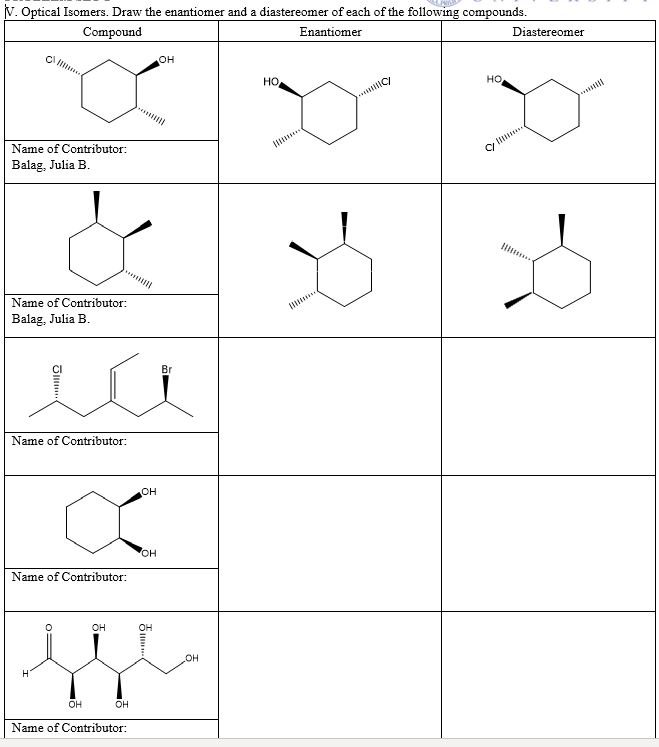
Solved V Optical Isomers Draw The Enantiomer And A Chegg Com

Draw A Diastereomer For Each Of The Following Compounds Study Com

Draw A Diastereomer For Each Of The Following Compounds Study Com
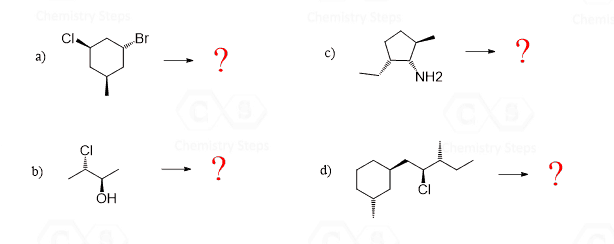
Enantiomers Diastereomers Identical Or Constitutional Isomers Chemistry Steps
0 comments
Post a Comment